Thermodynamics is a scientific branch that deals with the study of energy and its transformations. This branch of physics provides a framework for understanding and analyzing the behavior of systems. It ranges from microscopic particles to large-scale processes. Two key concepts in thermodynamics are entropy and enthalpy. They play crucial roles in understanding the behavior of energy in different systems. What is the difference between entropy vs enthalpy, are they different?
See also: Binary Cross Entropy vs Categorical Cross Entropy
Now, we will look at entropy and enthalpy, explore their definitions, measurements, application, and the differences between entropy vs enthalpy. We will compare these two concepts to have a better understanding of their unique characteristics and how they interact within thermodynamic systems.
Basic Concepts of Thermodynamics
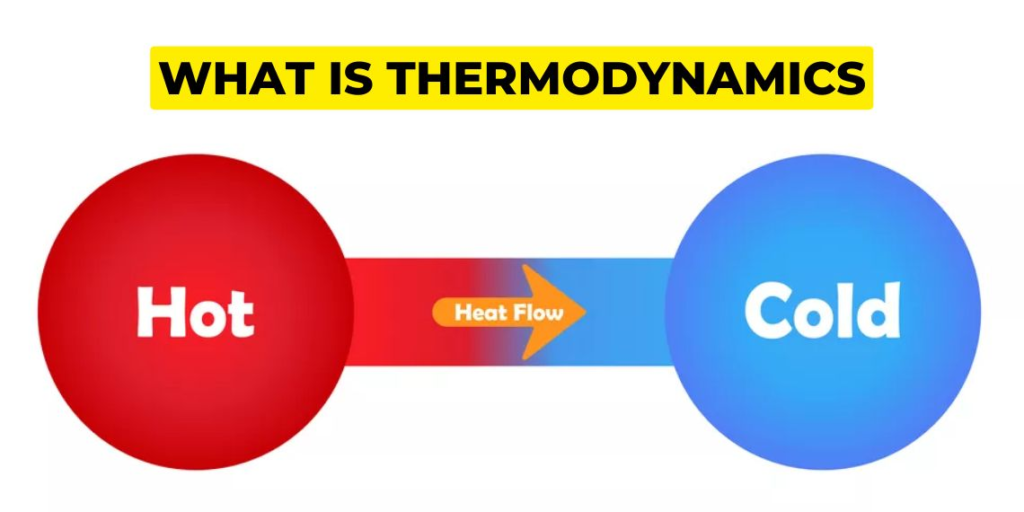
Thermodynamics is the study of energy and its transformations in various systems. It provides a framework for understanding energy behavior and the relationships between different forms of energy. It is essential to have a solid foundation in the basic principles of thermodynamics to grasp the concepts of entropy and enthalpy.
Laws of Thermodynamics
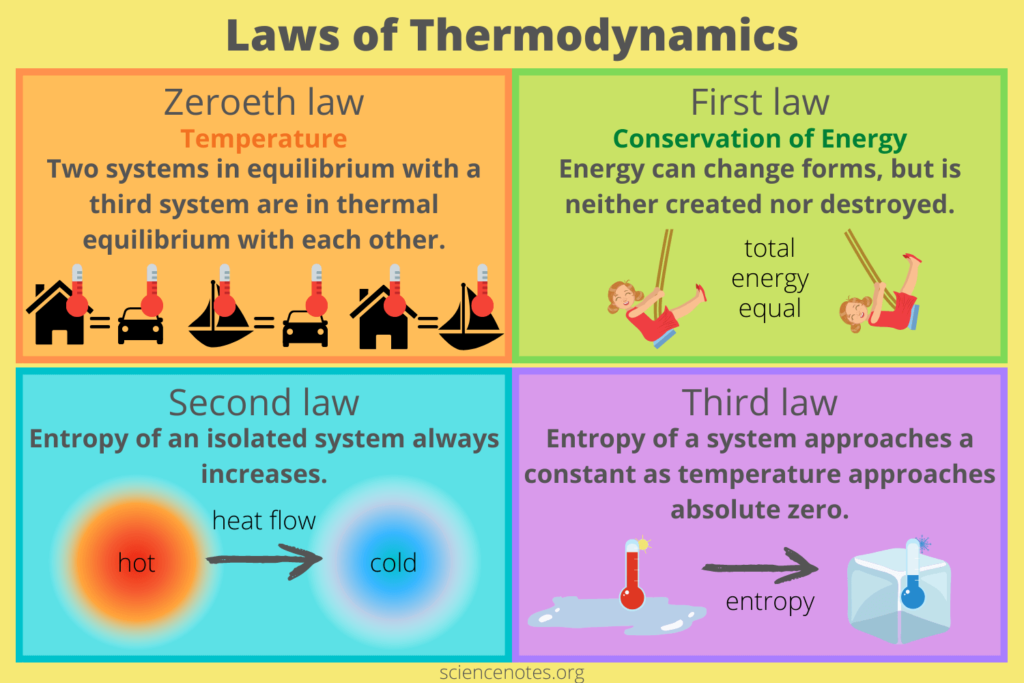
The laws of thermodynamics form the foundation of the entire field. These laws are fundamental principles that govern energy behavior in different systems. They provide a set of rules that we can’t violate and are universally applicable. The four laws of thermodynamics are:
- The Zeroth Law of Thermodynamics: This law establishes the thermal equilibrium concept and the transitive property of temperature. It says that if two of these systems are in thermal equilibrium along with a third system, then they must be in thermal equilibrium with each other.
- The First Law of Thermodynamics (Conservation of Energy): This law says that we or anything else can’t create energy. It can only be transferred or converted from one form to another. The total energy of an isolated system doesn’t change and is always the same.
- The Second Law of Thermodynamics: This law introduces the concept of entropy and defines the direction of energy flow. The second Law of Thermodynamics says that an isolated system’s entropy always increases or remains constant over time. It also introduces the concept of heat transfer and the impossibility of achieving 100% efficiency in energy conversions.
- The Third Law of Thermodynamics: This law deals with the behavior of systems at absolute zero temperature. It says that the more temperature is close to absolute zero, the more the entropy of a pure crystalline substance approaches zero.
Energy and Its Forms
Energy is a fundamental concept in thermodynamics. It is the ability of a system to do work or cause a change. Energy can exist in various forms, including:
- Kinetic Energy: It is the energy of the motion of an object.
- Potential Energy: The energy that stays in an object because of its position or condition.
- Thermal Energy: The temperature of an object or a system.
- Chemical Energy: The energy stored within the bonds of chemical compounds.
- Electrical Energy: The energy associated with the flow of electric charge.
- Nuclear Energy: The energy that the system releases during nuclear reactions.
Understanding these various energy forms and how we can convert or transfer them is essential in comprehending the concepts of entropy and enthalpy.
System and Surroundings
In thermodynamics, a system is the specific portion of the universe that is under consideration, while the surroundings encompass everything outside the system. The system can be an isolated system, closed system, or open system, depending on the exchange of energy and matter with the surroundings.
Understanding the distinction between the system and its surroundings is essential, as entropy and enthalpy are properties that describe the behavior and transformations within a system.
State Variables and Equilibrium
In thermodynamics, state variables are properties that describe the state of a system. Examples of state variables include temperature, pressure, volume, and composition. These variables are crucial in determining the thermodynamic behavior of a system.
Equilibrium is a state in which the system is stable and does not undergo any further changes. Understanding the concept of equilibrium is important since we often discuss entropy and enthalpy in relation to equilibrium states.
By grasping the basic concepts of thermodynamics, including the laws, forms of energy, system and surroundings, state variables, and equilibrium, we can lay a solid foundation for understanding entropy and enthalpy and their significance in the world of thermodynamics.
Defining and Understanding Entropy
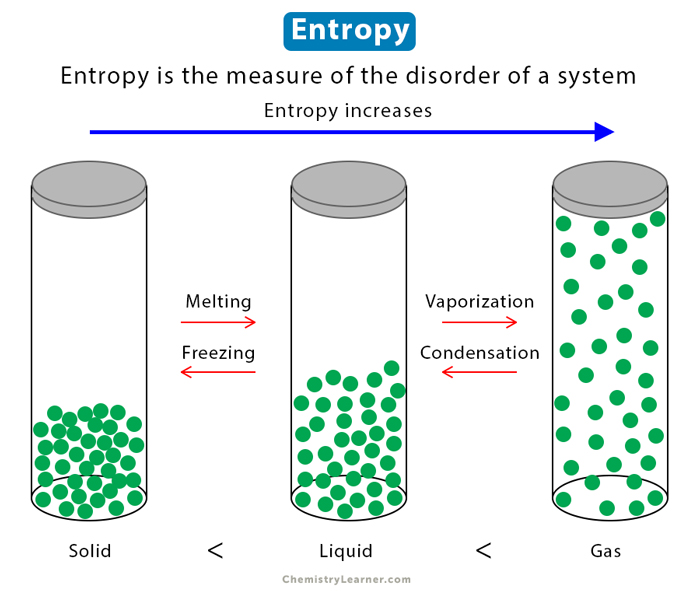
Entropy is a concept in thermodynamics that allows us to understand the behavior of energy in various systems. It is a measure of disorder or randomness within a system.
What is Entropy?
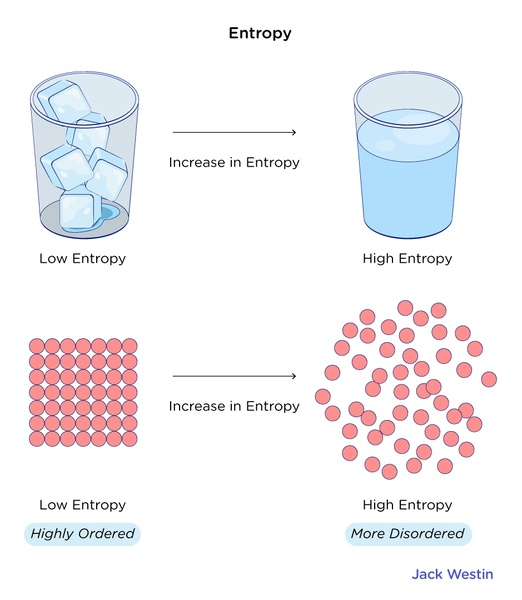
Entropy is basically the measure of the randomness or chaos within a system. It quantifies the number of possible microscopic arrangements that correspond to a given macroscopic state. In simpler terms, entropy provides information about how energy is distributed and dispersed in a system.
The Role of Entropy in Thermodynamics
Entropy has a close relationship with the second law of thermodynamics. As we mentioned before, this law says that entropy always increases or stays constant in an isolated system. This law implies that natural processes tend to move towards states of higher entropy, leading to a greater dispersion and equilibration of energy.
Entropy helps us understand why certain processes occur spontaneously, and others do not. In nature, systems tend to evolve towards states with maximum entropy because these states are more probable. Understanding entropy allows us to predict and analyze the behavior of energy in a wide range of systems, from microscopic particles to large-scale processes.
How We Measure Entropy
Entropy is a concept that can be challenging to quantify directly. However, we can calculate it by using mathematical formulas and measurements based on the properties of the system.
In thermodynamics, we generally use the symbol “S” to represent entropy. We can determine the entropy of a system by using different methods. IT generally depends on the system’s characteristics and the available information. Some common approaches to measure entropy include:
- Statistical Mechanics: Statistical mechanics provides a framework for calculating entropy based on the microscopic properties of particles within a system. By considering the probabilities of different microstates, statistical mechanics allows us to derive the macroscopic properties, including entropy.
- Entropy Changes: We can calculate entropy changes by evaluating the differences in entropy between the initial and final states of a system. This can be done by considering the heat transfer, temperature changes, and reversible processes involved.
- Entropy Tables and Charts: In some cases, entropy values for specific substances or systems are tabulated or charted in reference materials. These tables and charts provide convenient reference points for determining the entropy of a given system.
It is important to note that the measurement of entropy is context-dependent and requires a thorough understanding of the system’s characteristics and properties. We use various mathematical tools and techniques to calculate or estimate entropy, depending on the complexity of the system under consideration.
By comprehending the definition of entropy, its role in thermodynamics, and the methods used to measure it, we can begin to unravel the mysteries of this fundamental concept. In the next sections, we will further explore entropy by examining its relationship with energy and its comparison to another essential thermodynamic concept, enthalpy.
Defining and Understanding Enthalpy
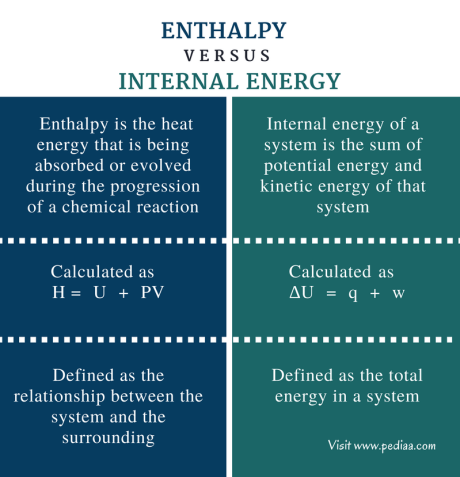
Enthalpy is another important concept in thermodynamics that describes the total energy of a system. It includes its internal energy and the energy exchanged with its surroundings.
What is Enthalpy?
Enthalpy is a property in thermodynamics, and it represents the total energy content of a system. It includes both the internal energy of the system (the energy associated with its molecular structure and temperature) and the energy exchanged with the surroundings (in the form of heat or work). We show Enthalpy by the symbol “H,” which we often express in units of joules or calories.
Enthalpy is a state function, meaning it depends only on the initial and final states of the system, regardless of the path taken to reach those states. It gives us extremely important information about the energy changes that occur during processes such as chemical reactions, phase transitions, and physical transformations.
The Role of Enthalpy in Thermodynamics
Enthalpy plays a crucial role in thermodynamics, particularly in the study of energy transfer and transformations. It allows us to quantify and analyze the heat flow associated with a system, as well as the work done by or on the system.
Enthalpy is particularly useful in the study of chemical reactions. By measuring the change in enthalpy (∆H) during a chemical reaction, we can see whether the reaction releases or absorbs energy. This information is vital for understanding the thermodynamics and feasibility of chemical processes.
How We Measure Enthalpy
We typically measure enthalpy using calorimetry, a technique that involves measuring heat changes associated with a system. Calorimeters are devices to accurately measure the heat flow between a system and its surroundings. By quantifying the heat that changes hands, we can calculate the change in enthalpy.
We can measure the enthalpy change (∆H) in different ways, depending on the specific system and conditions. Some common methods used to measure enthalpy are below.
- Bomb Calorimetry: This is the method we use to measure the enthalpy change of combustion reactions. The reaction takes place in a bomb calorimeter, which is a sealed container surrounded by water. The heat released or absorbed during the reaction is transferred to the water, and the temperature change is measured to determine the enthalpy change.
- Differential Scanning Calorimetry (DSC): Scanning Calorimetry measures the enthalpy changes associated with phase transitions, such as melting or crystallization. The sample is subjected to controlled temperature changes, and the heat flow is measured to determine the enthalpy change at various temperatures.
- Enthalpy Changes in Solution: Enthalpy changes during dissolution or mixing of substances can be measured using techniques such as solution calorimetry. By measuring the temperature change associated with the dissolution process, the enthalpy change can be determined.
These methods, along with others specific to different systems, allow scientists and engineers to measure and quantify enthalpy changes in various processes and reactions.
Comparing Entropy vs Enthalpy
Entropy and enthalpy are two essential concepts in thermodynamics that provide valuable insights into the behavior of energy within systems.
Similarities between Entropy vs Enthalpy
While entropy and enthalpy are distinct concepts, they do share some similarities. Both entropy and enthalpy:
- Both are state functions. Both entropy and enthalpy are state functions, meaning they don’t depend on the path taken to reach certain states but only on the initial and final states of the system. This allows for the calculation of changes in entropy (∆S) and enthalpy (∆H) by considering the initial and final states of a system.
- Both are extensive properties: Both entropy and enthalpy are extensive properties, meaning they depend on the size or amount of the system. For example, the entropy and enthalpy of a given substance will increase as the quantity or mass of that substance increases.
- Are related to energy: Both entropy and enthalpy are related to the energy of a system. Entropy describes the distribution and dispersal of energy within a system, while enthalpy represents the total energy content of the system, including its internal energy and energy exchanged with the surroundings.
Differences between Entropy vs Enthalpy
Entropy and enthalpy have similar characteristics, but this doesn’t mean that they are all the same. They also have different characteristics that set them apart. Here are some key differences between entropy and enthalpy.
- Definition: Entropy is a measure of the disorder or randomness within a system, while enthalpy is a measure of the total energy of a system, including its internal energy and energy exchanged with the surroundings.
- Symbol: Entropy is commonly represented by the symbol “S,” while enthalpy is represented by the symbol “H.”
- Units: Entropy is typically measured in units of joules per Kelvin (J/K) or calories per Kelvin (cal/K), while enthalpy is measured in units of joules (J) or calories (cal).
- Calculation: Entropy can be calculated based on the probabilities of different microstates in a system, while enthalpy is calculated based on the heat transfer and work done on or by the system.
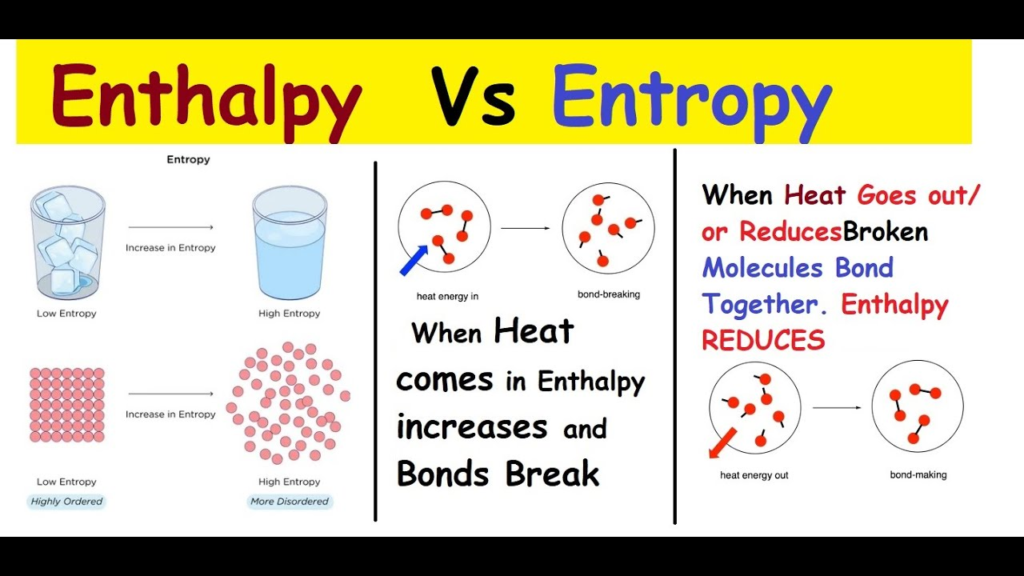
How Entropy and Enthalpy Interact In Thermodynamic Systems
Entropy and enthalpy are interconnected and interact within thermodynamic systems. According to the second law of thermodynamics, the entropy of an isolated system always increases or doesn’t change. As a result, energy tends to disperse and equilibrate, leading to an increase in entropy.
Enthalpy changes (∆H) can affect the entropy change (∆S) of a system and vice versa. For example, in an exothermic reaction where heat is released, the entropy change (∆S) of the system may increase due to the dispersal of energy. Conversely, in an endothermic reaction where heat is absorbed, the entropy change (∆S) of the system may decrease due to the concentration of energy.
We can predict and analyze the behavior of energy within a thermodynamic system by understanding the interaction between entropy and enthalpy. It provides insights into the feasibility and directionality of processes and reactions.
By comparing entropy and enthalpy, we gain a deeper understanding of their unique characteristics and their roles in thermodynamics.
Real-World Applications of Entropy and Enthalpy
Entropy and enthalpy, as fundamental concepts in thermodynamics, have numerous real-world applications in various fields.
Use of Entropy in Engineering and Design
Entropy plays a crucial role in engineering and design, particularly in optimizing processes and systems. Some key applications include:
- Energy Efficiency: Understanding entropy allows engineers to design more efficient systems by minimizing energy losses due to heat transfer and other irreversible processes. By analyzing entropy changes, engineers can identify areas of high entropy generation and develop strategies to reduce them.
- Heat Exchangers: These devices transfer heat from one fluid to another. The design and efficiency of heat exchangers depend on understanding entropy changes and maximizing heat transfer while minimizing pressure losses.
- Refrigeration and Air Conditioning: Entropy helps in the design and analysis of refrigeration and air conditioning systems. By considering entropy changes during the refrigeration cycle, engineers can optimize system performance and ensure efficient cooling.
Use of Enthalpy in Heating and Cooling Systems
Enthalpy is of significant importance in heating and cooling systems, where energy transfer and transformations are crucial. Some notable applications include:
- HVAC Systems: Enthalpy is used to quantify the total energy content of air and determine the heating or cooling requirements in HVAC (Heating, Ventilation, and Air Conditioning) systems. By considering enthalpy changes, engineers can design and optimize systems for comfort and energy efficiency.
- Heat Pumps: Enthalpy calculations are essential in heat pump systems, which transfer heat from a lower temperature source to a higher temperature sink. By evaluating enthalpy changes, engineers can determine the energy efficiency and performance of heat pumps.
- Thermal Power Plants: Enthalpy plays a critical role in thermal power plants, where heat energy is converted into electrical energy. By analyzing enthalpy changes in different stages of the power plant, engineers can optimize the conversion process and maximize energy output.
How Entropy and Enthalpy Guide Chemical Reactions
Entropy and enthalpy are key considerations in chemical reactions, providing insights into the thermodynamics and feasibility of processes. Some notable applications include:
- Reaction Spontaneity: The entropy change (∆S) and enthalpy change (∆H) of a reaction determine its spontaneity. By considering the relationship between entropy and enthalpy (∆G = ∆H – T∆S), chemists can predict if there will be a random reaction under specific conditions.
- Phase Transitions: Entropy and enthalpy changes, such as melting, boiling, and condensation, play a significant role in phase transitions. By analyzing these changes, scientists can understand the energy requirements and behavior of substances during phase transitions.
- Chemical Equilibrium: Entropy and enthalpy changes influence the position of chemical equilibrium. By considering the Gibbs free energy (∆G = ∆H – T∆S) and the relationship between entropy and enthalpy, chemists can determine the conditions that favor either the forward or reverse reaction.
These are just a few examples of the real-world applications of entropy and enthalpy. Their understanding and utilization are essential in various fields, including engineering, design, heating and cooling systems, and chemical reactions. By harnessing the principles of entropy and enthalpy, scientists and engineers can optimize processes, improve efficiency, and advance technological advancements.
Conclusion
In the expansive realm of thermodynamics, entropy and enthalpy stand as pillars guiding our understanding of energy and its transformations. This post looked at their distinct roles and compared entropy vs enthalpy to understand better what they actually entail. Entropy is the measure of disorder, and enthalpy is the gauge of total energy within systems.
The interconnectedness of entropy vs enthalpy within thermodynamic systems unveils their pivotal roles in steering real-world applications. From engineering designs enhancing energy efficiency to the prediction and analysis of chemical reactions, these concepts serve as guiding lights, shaping innovations across diverse fields.